Categories: Featured Articles » Novice electricians
Number of views: 52646
Comments on the article: 4
Transistors Part 2. Conductors, insulators and semiconductors
Beginning of the article: Transistor history, Transistors: purpose, device and principles of operation
 In electrical engineering, various materials are used. The electrical properties of substances are determined by the number of electrons in the outer valence orbit. The fewer electrons are in this orbit, the weaker they are associated with the nucleus, the easier they can go to travel.
In electrical engineering, various materials are used. The electrical properties of substances are determined by the number of electrons in the outer valence orbit. The fewer electrons are in this orbit, the weaker they are associated with the nucleus, the easier they can go to travel.
Under the influence of temperature fluctuations, the electrons break away from the atom and move in the interatomic space. Such electrons are called free, and they create an electric current in the conductors. Is there a large interatomic space, is there room for free electrons to travel inside matter?
The structure of solids and liquids seems continuous and dense, reminiscent in structure of a ball of thread. But in fact, even solids are more like a fishing or volleyball net. Of course, this cannot be discerned at the household level, but it has been established by accurate scientific studies that the distances between the electrons and the nucleus of atoms are much larger than their own dimensions.
If the size of the atomic nucleus is represented in the form of a ball the size of a soccer ball, then the electrons in this model will be the size of a pea, and each such pea is located from the "core" at a distance of several hundred and even thousands of meters. And between the nucleus and the electron is emptiness - there is simply nothing! If we imagine the distances between the atoms of matter on the same scale, the dimensions will turn out to be fantastic at all - tens and hundreds of kilometers!
Good conductors of electricity are metals. For example, the atoms of gold and silver have only one electron in the outer orbit, therefore they are the best conductors. Iron also conducts electricity, but slightly worse.
Conduct electricity even worse high resistance alloys. These are nichrome, manganin, constantan, fechral and others. Such a variety of high-resistance alloys is due to the fact that they are designed to solve various problems: heating elements, strain gauges, reference resistors for measuring instruments, and much more.
In order to evaluate the ability of a material to conduct electricity, the concept of "Electrical conductivity". The return value is resistivity. In mechanics, these concepts correspond to the specific gravity.
Insulators, unlike conductors, are not inclined to lose electrons. In them, the bond of the electron with the nucleus is very strong, and there are almost no free electrons. More precisely, but very few. At the same time, in some insulators there are more of them, and their insulation quality is, accordingly, worse. It is enough to compare, for example, ceramics and paper. Therefore, insulators can conditionally be divided into good and bad.
The appearance of free charges even in insulators is caused by thermal vibrations of electrons: under the influence of high temperature, the insulating properties deteriorate, some electrons still manage to break away from the nucleus.
Similarly, the resistivity of an ideal conductor would be zero. But fortunately there is no such conductor: imagine what Ohm's law ((I = U / R) would look like with zero in the denominator !!! Farewell to mathematics and electrical engineering.
And only at a temperature of absolute zero (-273.2 ° C) the thermal fluctuations completely stop, and the worst insulator becomes good enough. In order to determine numerically “this” is bad - good use the concept of resistivity. This is the resistance in Ohms of a cube with an edge length of 1 cm, the dimension of resistivity is obtained in ohms / cm. The specific resistance of some substances is shown below.Conductivity is the reciprocal of the resistivity, is the unit of measure of Siemens, - 1Sm = 1 / Ohm.
They have good conductivity or low resistivity: silver 1.5 * 10 ^ (- 6), read how (one and a half to ten to the power minus six), copper 1.78 * 10 ^ (- 6), aluminum 2.8 * 10 ^ (- 6). The conductivity of alloys with high resistance is much worse: constantan 0.5 * 10 ^ (- 4), nichrome 1.1 * 10 ^ (- 4). These alloys can be called bad conductors. After all these complex numbers, substitute Ohm / cm.
Further, semiconductors can be distinguished as a separate group: germanium 60 Ohm / cm, silicon 5000 Ohm / cm, selenium 100 000 Ohm / cm. The resistivity of this group is greater than that of bad conductors, but less than that of bad insulators, not to mention good ones. Probably, with the same success, semiconductors could be called semi-insulators.
After such a short acquaintance with the structure and properties of an atom, one should consider how atoms interact with each other, how atoms interact with each other, how molecules are made of them, from which various substances are composed. To do this, you will again have to remember the electrons in the outer orbit of the atom. After all, it is they who participate in the bonding of atoms into molecules and determine the physical and chemical properties of matter.
How atoms are made from atoms
Any atom is in a stable state if there are 8 electrons in its outer orbit. He does not seek to take electrons from neighboring atoms, but he does not give up his own. To verify this, it is enough in the periodic table to look at inert gases: neon, argon, krypton, xenon. Each of them has 8 electrons in the outer orbit, which explains the reluctance of these gases to enter into any relations (chemical reactions) with other atoms, to build molecules of chemical substances.
The situation is quite different for those atoms that do not have 8 cherished electrons in their outer orbit. Such atoms prefer to unite with others in order to supplement their outer orbit with up to 8 electrons and find a calm stable state.
For example, the well-known water molecule H2O. It consists of two hydrogen atoms and one oxygen atom, as shown in the figure. 1.
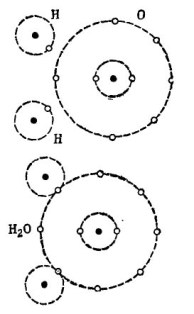
Picture 1. How a water molecule is created.
In the upper part of the figure, two hydrogen atoms and one oxygen atom are shown separately. There are 6 electrons in the outer orbit of oxygen and two electrons at two hydrogen atoms are nearby. Oxygen until the cherished number 8 is missing just two electrons in the outer orbit, which he will receive by adding two hydrogen atoms to himself.
Each hydrogen atom lacks 7 electrons in its outer orbit for complete happiness. The first hydrogen atom receives in its outer orbit 6 electrons from oxygen and another electron from its twin - the second hydrogen atom. There are now 8 electrons in its outer orbit along with its electron. The second hydrogen atom also completes its outer orbit to the cherished number 8. This process is shown in the lower part of the figure. 1.
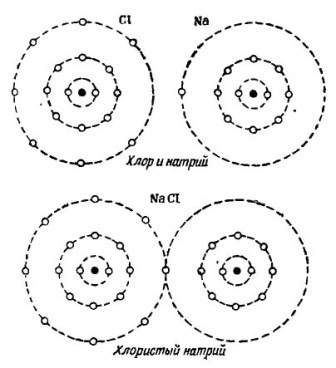
On the image 2 The process of combining sodium and chlorine atoms is shown. The result is sodium chloride, which is sold in stores called salt.
Picture 2. The process of combining sodium and chlorine atoms
Here, too, each of the participants receives the missing number of electrons from the other: chlorine attaches a single sodium electron to its own seven electrons, while it gives its atoms to the sodium atom. Both atoms in the outer orbit have 8 electrons, which is where full agreement and prosperity are achieved.
Valency of atoms
Atoms with 6 or 7 electrons in their outer orbit tend to attach 1 or 2 electrons to themselves. They say about such atoms that they are one or divalent. But if in the outer orbit of an atom 1, 2 or 3 electrons, then such an atom tends to give them away. In this case, the atom is considered one, two or trivalent.
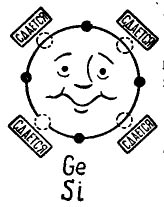
If there are 4 electrons in the outer orbit of an atom, then such an atom prefers to combine with the same one, which also has 4 electrons. This is how germanium and silicon atoms used in the production of transistors combine. In this case, the atoms are called tetravalent. (The atoms of germanium or silicon can be combined with other elements, for example, oxygen or hydrogen, but these compounds are not interesting in the plan of our story.)
On the image 3 a germanium or silicon atom is shown which wishes to combine with the same atom. Small black circles are the atom’s own electrons, and light circles indicate the places where the electrons of the four atoms - neighbors - fall.
Picture 3. Atom of germanium (silicon).
The crystal structure of semiconductors
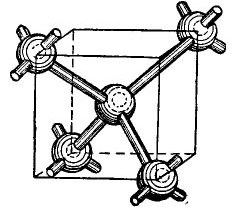
The germanium and silicon atoms in the periodic table are in the same group as carbon (the chemical formula of diamond C is simply large carbon crystals obtained under certain conditions), and therefore, when combined, form a diamond-like crystalline structure. The formation of such a structure is shown, in a simplified, of course, form in the figure 4.
Picture 4.
In the center of the cube is a germanium atom, and 4 more atoms are located in the corners. The atom depicted in the center of the cube is bound by its valence electrons to its nearest neighbors. In turn, the angular atoms give their valence electrons to the atom located in the center of the cube and its neighbors - atoms not shown in the figure. Thus, the outer orbits are supplemented by up to eight electrons. Of course, there is no cube in the crystal lattice, it is just shown in the figure so that the mutual, volumetric arrangement of atoms is clear.
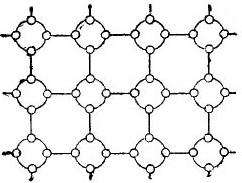
But in order to simplify the story about semiconductors as much as possible, the crystal lattice can be depicted in the form of a flat schematic drawing, despite the fact that the interatomic bonds are nevertheless located in space. Such a circuit is shown in the figure. 5.
Picture 5. The germanium crystal lattice in a flat form.
In such a crystal, all the electrons are firmly attached to the atoms by their valence bonds, therefore, apparently, there are simply no free electrons here. It turns out that in front of us is an insulator in the figure, since there are no free electrons in it. But actually it is not.
Intrinsic conductivity
The fact is that under the influence of temperature, some electrons still manage to break away from their atoms, and for some time free themselves from the bond with the nucleus. Therefore, a small amount of free electrons in a germanium crystal exists, due to which it is possible to conduct an electric current. How many free electrons exist in a germanium crystal under normal conditions?
There are no more than two such free electrons per 10 ^ 10 (ten billion) atoms, so germanium is a poor conductor, or as is customary to say a semiconductor. It should be noted that only one gram of germanium contains 10 ^ 22 (ten thousand billion billion) atoms, which allows you to "get" about two thousand billion free electrons. It seems that enough to pass a large electric current. To deal with this issue, it is enough to recall what a current of 1 A.
A current of 1 A corresponds to passing through a conductor in one second an electric charge of 1 Coulomb, or 6 * 10 ^ 18 (six billion billion) electrons per second. Against this background, two thousand billion free electrons, and even scattered over a huge crystal, are unlikely to ensure the passage of high currents. Although, due to thermal motion, small conductivity exists in Germany. This is the so-called intrinsic conductivity.
Electronic and hole conductivity
As the temperature rises, additional energy is transferred to the electrons, their thermal vibrations become more energetic, as a result of which some electrons manage to break away from their atoms.These electrons become free and, in the absence of an external electric field, make chaotic motions and move in free space.
Atoms that have lost electrons cannot make random movements, but only slightly oscillate relative to their normal position in the crystal lattice. Such atoms, which have lost electrons, are called positive ions. We can assume that in place of electrons torn from their atoms, free spaces are obtained, which are commonly called holes.
In general, the number of electrons and holes is the same, so a hole can capture an electron that is nearby. As a result, an atom from a positive ion again becomes neutral. The process of combining electrons with holes is called recombination.
At the same frequency, electrons are separated from atoms, therefore, on average, the number of electrons and holes for a particular semiconductor is equal to, is constant and dependent on external conditions, especially temperature.
If a voltage is applied to the semiconductor crystal, then the electron motion will be ordered, a current will flow through the crystal due to its electron and hole conductivity. This conductivity is called intrinsic, it was already mentioned a little higher.
But pure semiconductors with electronic and hole conductivity are unsuitable for the manufacture of diodes, transistors, and other details, since the basis of these devices is the p-n (read “pe-en”) junction.
To obtain such a transition, two types of semiconductors are needed, two types of conductivity (p - positive - positive, hole) and (n - negative - negative, electronic). These types of semiconductors are obtained by doping, adding impurities to pure germanium or silicon crystals.
Although the amount of impurities is very small, their presence to a large extent changes the properties of the semiconductor, allows you to get semiconductors of different conductivity. This will be discussed in the next part of the article.
Boris Aladyshkin, https://e.imadeself.com/en
See also at e.imadeself.com
: