Categories: Interesting Facts, Electrician at home
Number of views: 136933
Comments on the article: 16
Why can not connect copper and aluminum in the wiring?
 What is in electrical engineering Do not directly connect copper and aluminum conductorsis not a secret even for many ordinary people who have no relation to the electrician. On the part of the same inhabitants, professional electricians often ask: “Why?”.
What is in electrical engineering Do not directly connect copper and aluminum conductorsis not a secret even for many ordinary people who have no relation to the electrician. On the part of the same inhabitants, professional electricians often ask: “Why?”.
Pochemochki of any age can drive anyone into a dead end. Here is a similar case. A typical professional answer: “Why, why ... Because it will burn. Especially if the current is high. " But this does not always help. Since this is often followed by another question: “Why will it burn? Why does copper with steel not burn, aluminum with steel not burn, and aluminum with copper burn? ”
To the last question you can hear different answers. Here are some of them:
1) Aluminum and copper have different coefficients of thermal expansion. When a current passes through them, they expand differently; when the current stops, they cool differently. As a result, a series of expansion-contractions changes the geometry of the conductors, and the contact becomes loose. And then in place bad contact heating occurs, it deteriorates even more, an electric arc appears, which completes the whole thing.
2) Aluminum forms an oxide non-conductive film on its surface, which worsens contact from the very beginning, and then the process proceeds along the same growing line: heating, further deterioration of contact, arc and destruction.
3) Aluminum and copper form a “galvanic pair”, which simply cannot but overheat at the point of contact. And again, heating, arc, and so on.
Where is the truth, after all? What happens there, at the junction of copper and aluminum?
The first of the answers given is still untenable. Here are the tabular data on the linear coefficient of thermal expansion for metals used for electrical installation: copper - 16.6 * 10-6m / (m * gr. Celsius); aluminum - 22.2 * 10-6m / (m * g. Celsius); steel - 10.8 * 10-6m / (m * g. Celsius).
Obviously, if it were expansion coefficients, the most unreliable contact would be between a steel and aluminum conductor, because their expansion coefficients differ by half.
But even without tabular data, it is clear that differences in linear thermal expansion are relatively easily compensated by the use of reliable clamps that create constant pressure on the contact. To expand the metals, compressed, for example, using a well-tightened bolted connection, remains only to the side, and temperature changes are not able to seriously weaken the contact.
The oxide film option is also not entirely true. After all, this same oxide film allows you to connect aluminum conductors with steel and with other aluminum conductors. Yes, of course, the use of a special lubricant against oxides is recommended, yes, a systematic revision of compounds involving aluminum is recommended. But all this is allowed and works for years.
But the version with a galvanic pair really has a right to exist. But here, all the same, it cannot do without oxides. After all, a copper conductor is also quickly coated with oxide, with the only difference being that copper oxide conducts current more or less.
But if a copper and aluminum conductor are connected, their oxides have the possibility of dissociation, that is, decay into charged ions. Dissociation is possible due to natural moisture, which is always in the air. Ions of aluminum and copper oxides, being particles with different electric potentials, begin to take part in the current flow. The process known as “electrolysis” begins (see - Electrolysis application).
During electrolysis, ions transfer charges and move themselves. But, in addition, ions are particles of metal conductors.When they move, the metal is destroyed, shells and voids are formed. This is especially true for aluminum. Well, and where there are voids and sinks, it is no longer possible to have reliable electrical contact. Bad contact starts to warm up, it gets worse, and so on up to a fire.
Note that the wetter the surrounding air, the more intensively all of these processes occur. And the uneven thermal expansion and the non-conductive layer of aluminum oxide are just aggravating factors, nothing more.
In addition to the article, there is a useful plate that clearly shows the compatibility and incompatibility of individual metals and alloys when they are combined. Copper and aluminum can not be interconnected, since they are incompatible.
Compatibility of some metals and alloys
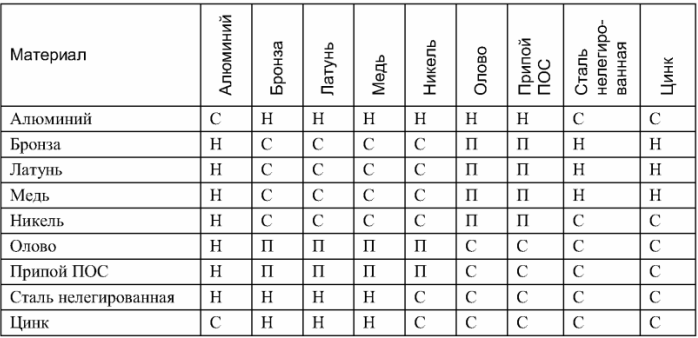
Note: C - compatible, H - incompatible, P - compatible when soldering, with direct connection form a galvanic pair.
See also at e.imadeself.com
:
