Categories: Featured Articles » Interesting Facts
Number of views: 13713
Comments on the article: 2
Unexpected properties of familiar carbon
 Fundamental science in recent years has rarely spoiled engineers with discoveries suitable for industrial implementation. But the last two decades have become a pleasant exception. Two Nobel Prizes and a third discovery, not yet evaluated by the Nobel Committee, created prospects for engineers and technologists to work for many decades. And they are all associated with one element - carbon.
Fundamental science in recent years has rarely spoiled engineers with discoveries suitable for industrial implementation. But the last two decades have become a pleasant exception. Two Nobel Prizes and a third discovery, not yet evaluated by the Nobel Committee, created prospects for engineers and technologists to work for many decades. And they are all associated with one element - carbon.
Why are these discoveries so important and what new can such a familiar chemical element give us? Everything that we know about carbon makes it the most interesting and important element of the periodic table. To begin with, life itself arose on a carbon basis. The ability of carbon atoms to combine into complex structures has allowed nature to create protein organisms and breathe the mind into humans. All organic chemistry, with its abundance of new, artificial materials, is based on complex compounds of carbon with other elements.
But atomic carbon is no less surprising than its compounds. This is the only element that has so far 8 allotropic (various) modifications. The word "bye" is quite appropriate, because carbon can bring more surprises in the future. Just a few decades ago, we knew only two carbon modifications: graphite and diamond.
We had and have to deal with graphite, drawing with a pencil on paper. A diamond is an exhilarating brilliance of diamonds and a symbol of wealth. True, the development of technology has led to the recognition of another property of diamond - its unsurpassed hardness. And now, despite the complexity of the processes, artificial diamonds are synthesized and widely used in technology. Hard rock drilling, stone cutting wheels, grinding pastes - you can long list the uses of this carbon modification.
 But between a very hard state and a very soft state lies an abyss. It was surprising that such an important element on which all modern chemistry is based has only two such antagonistic states.
But between a very hard state and a very soft state lies an abyss. It was surprising that such an important element on which all modern chemistry is based has only two such antagonistic states.
And finally, in the last century, at the beginning of the 60s, the third modification of carbon was discovered - carbin. This is a chain of interconnected carbon atoms. The discovery was made by chemists, and did not receive much resonance, since it did not find practical application.
And here is the next form of carbon - fullerenes aroused much greater interest. Carbon atoms assembled into a spherical molecule opened the way to the creation of completely new materials and compounds. For opening fullerene carbon modification a group of scientists in 1996 awarded the Nobel Prize
Already, fullerenes are used in the pharmaceutical industry to create anti-cancer drugs, in radio electronics, and in the automotive industry as additives for motor oils. The list of applications is remarkably wide, but the prospects for future use are even broader.
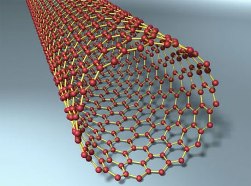
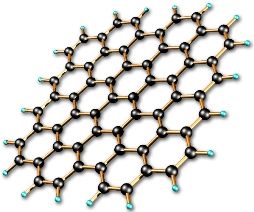
 Further discoveries related to carbon fell like a cornucopia. Lonsdaleit, found in meteorites and subsequently synthesized artificially, carbon nanotubes, graphene.
Further discoveries related to carbon fell like a cornucopia. Lonsdaleit, found in meteorites and subsequently synthesized artificially, carbon nanotubes, graphene.
We did not have time to master one allotropic form, as two more were unexpectedly added to it. And these forms of carbon will make it possible in the coming years to radically change both the communication structure of mankind and human life itself (read more about the prospects for using graphene and carbon nanotubes in the article about graphene electronics).
Thanks to the use of new carbon properties, there are unlimited possibilities for creating quantum computers, data transmission lines with a speed of more than 100 Gbit / s, sensors that read signals from a living cell, and much more,what science fiction writers did not dream about.
And it did not take long to wait: the prospects for the commercial use of discoveries are so great that dozens of companies are already demonstrating product samples that include carbon nanotubes and graphenes.
See also at e.imadeself.com
:
