Categories: Featured Articles » Interesting Facts
Number of views: 11242
Comments on the article: 1
What is rosin: composition, properties, application
Everyone who in the days of the Soviet Union dealt with a soldering iron knows firsthand the rosin. However, today, when soldering fluxes are used everywhere, rosin for soldering is used less and less. But rosin is used not only for soldering. Let's remember what rosin is in general, where it comes from and where else it is used.
Rosin or colophon resin got its name from the ancient Greek city of Colophon, where a special pine resin was highly valued by musicians in its time.
Rosin itself is a rather fragile amorphous substance of a vitreous structure with a characteristic glass luster. The color of the rosin can be from light yellow to dark red. As a component, it is found in coniferous resins and consists mainly of the carboxylic acids of the phenanthrene series and their isomers.
The raw material for the production of rosin was originally gum - a resinous substance secreted by conifers when they are mechanically damaged. Turpentine and other volatile substances, of which 25% in crude resin, were evaporated from gum.

In industrial production, rosin is obtained as an extract of shredded coniferous wood using an organic solvent or by distillation of crude tall oil, which is a waste product of the pulp and paper industry. Thus, depending on the method of preparation, rosin is pine, tall, etc.
Rosin is insoluble in water, but readily soluble in organic solvents such as ethyl alcohol, ether, acetone, chloroform and benzene. The melting point of rosin depends on the method of its preparation, and lies in the range from 50 to 130 ° C. The composition of rosin is always dominated by carboxylic (resin) acids, the main of which is abietic acid, which is also part of amber.
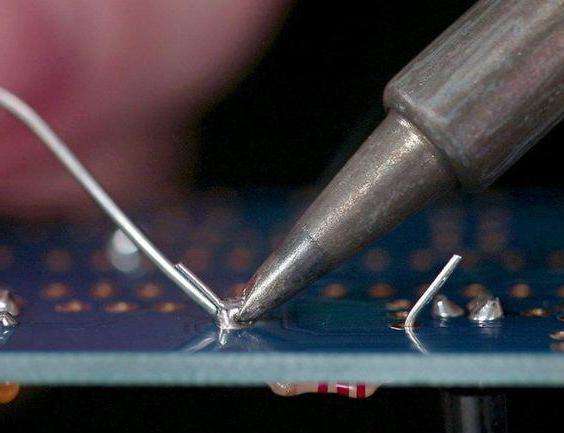
Rosin as a solder flux
To this day, rosin is widely used as a solder flux (to remove thin films of oxides from the surfaces of soldered metals) when soldering with fusible solders and tinning.
Thanks to the use of rosin during soldering, the wettability of solder board-mounted components is improved. When soldering parts from copper, copper alloys, steel, zinc and other non-ferrous metals except aluminum, rosin enjoys well-deserved popularity due to its efficiency.

In the molten state, rosin easily dissolves oxide films: oxidized metals are essentially partially reduced to metals, and partially are converted into low-melting salts.
At the same time, rosin is unstable to moisture. Under the influence of atmospheric moisture, it hydrolyzes, enhances the corrosion of the compounds with which it is in contact. Therefore, after soldering, rosin should be washed off (for example, with alcohol or acetone). Rosin is used as a dielectric unless in very tight devices.

For soldering, a weak solution of rosin in isopropyl or ethyl alcohol with the addition of glycerin is best suited. Such a solution is very effective, although it has only a slightly yellowish color. If it is too concentrated, that is, if there is too much rosin in the solution, then it will be difficult to wash it off the board, and the soldering iron tip will become more dirty.
By the way, for soldering highly contaminated or highly oxidized surfaces, rosin-alcohol-glycerin fluxes are ineffective. In such cases, it is recommended to use inorganic, for example, acid fluxes.In addition, glycerin, due to its hygroscopicity, promotes corrosion of conductors and electrical leaks on the surface of the dielectric of the board, therefore, the remains of the flux with glycerin should always be removed immediately after soldering and especially carefully.
Storage of rosin solutions requires a special approach to prevent the precipitation of crystals of resin acids. To do this, it is imperative that the filtered solution should not be stored in iron cans, since rust on their walls tends to form iron resins with resin acids, which contribute to the crystallization of resin acids. And in solutions with white spirit you need to add at least 4% turpentine.
Other uses of rosin
Rosin processing products are used for gluing cardboard and paper, it serves as an emulsifier in the production of artificial rubber, and is used in the production of plastics, rubbers, soap, linoleum, artificial leather, varnishes, paints, electrical insulating compounds and mastics.
The rods of musical instruments, the tip of a billiard cue, and also the shoes of ballerinas are rubbed with rosin to reduce slipping. In weightlifting, mountaineering, baseball, when practicing on the horizontal bar, rosin allows you to clearly capture grip without slipping, and there will be no calluses from this. It is crushed rosin used to simulate smoke in the cinema.

In the old days, when there were no effective tensioners, transmission belts of various mechanisms were rubbed with rosin. Even today, when starting mechanisms with high inertia, from time to time they resort to the use of rosin.
By adding rosin powder to a solid resin based varnish, you can increase its fluidity. Pure rosin at the base of the varnish makes it very soft and unstable, whitening with moisture, and easily washable. High-quality rosin-based varnishes are obtained by esterification (fusion) of 6% glycerol with rosin.
By the way, one of the rosin esters is registered as a food supplement E915 - pentadine resin. But practically as a food additive E915 is not used either in Russia or in the EU countries.
See also at e.imadeself.com
:
